Pharmacovigilance via VigiMobile app- "Protecting Patients Through Smart Reporting"
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. There are many available methods for reporting adverse effects of medicines and vaccines, but a much smarter and efficient way to report adverse events of medicines and vaccine is through VigiMobile app.
This app has made it possible for every single individual to report any adverse effect associated with any medicine or vaccine just by using its mobile.
The reporting system of VigiMobile app, is available in almost every country and the regulatory authority or Pharmacovigilance centres of a country reports the information, regarding the adverse events of medicines and vaccine, in the same manner.
- In order to understand the working mechansim, we take the example of Pakistan. Pharmacovigilance in Pakistan is executed by the National Pharmacovigilance Centre (NPC) under the division of pharmacy services, Drug Regulatory Authority of Pakistan (DRAP). NPC collects the reports of adverse drug reactions, that occur as a result of administration of medicines and vaccines, process and analyse these reports and submit them to global database designed for adverse event reports of medicines and vaccines- Vigibase, introduced by WHO and managed by Uppsala monitoring centre.
Uppsala Monitoring Centre (UMC):
Uppsala monitoring centre is a non-profit, self-funded and independent foundation that works with WHO (world health organization) as collaborative centre of WHO programme for International Drug Monitoring (WHO PIDM), with the vision of ensuring safer use of medicines and vaccines around the globe.
WHO programme for International Drug Monitoring (WHO PIDM):
WHO-PIDM is an international collaborative program developed with the aim of identifying medicine and vaccine related safety problem. This program being initiated by WHO, consists of 180 full members and associate members from all over the world and thus brings approximately 99% of world population on this platform.
How The Reports Are transferred from Patient to UMC?
So, in order to understand how a single report from a patient or health care profession reaches the Uppsala monitoring centre let’s look at the mediums involved in data transfer.
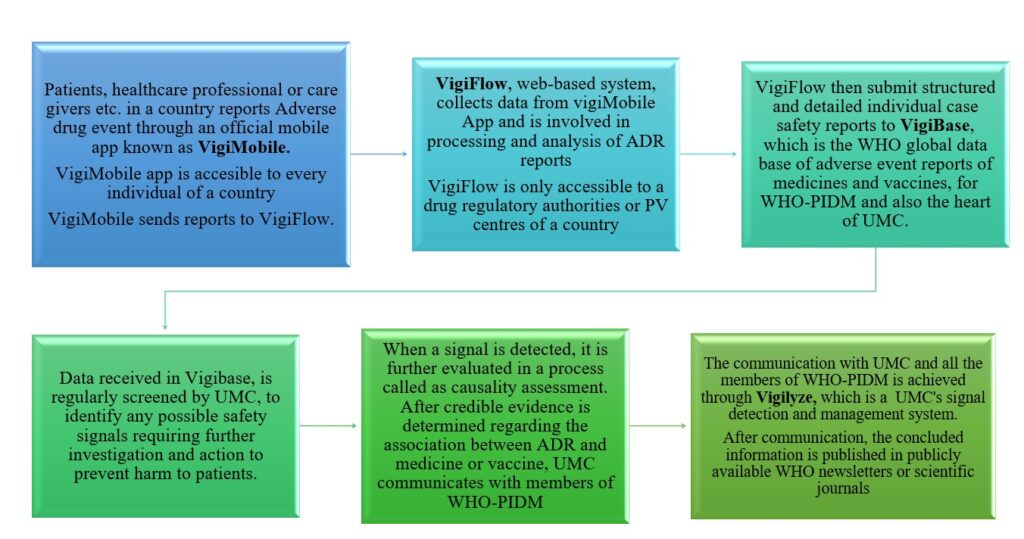
- Patients, healthcare professional or care givers etc. of a country, reports Adverse drug event through an official mobile app known as VigiMobile. VigiMobile app is accessible to every individual of a country. VigiMobile transfer reports to VigiFlow.
- VigiFlow is a web-based system that collects data from VigiMobile App and is involved in processing and analysis of Adverse event reports of medicines and vaccines. VigiFlow is only accessible to a drug regulatory authority or pharmacovigilance centres of a country.
- VigiFlow then submit structured and detailed individual case safety reports to VigiBase, which is the WHO global data base of adverse event reports of medicines and vaccines, for WHO-PIDM and is starting point of UMC’s working process.
- Data received in Vigibase, is regularly screened by UMC, to identify any possible safety signals requiring further investigation and action to prevent harm to patients.
- When a signal is detected, it is further evaluated in a process called as causality assessment. After credible evidence is determined regarding the association between Adverse drug event and a medicine or vaccine, UMC communicates with members of WHO-PIDM
- The communication between UMC and all the members of WHO-PIDM is achieved through Vigilyze, which is a UMC’s signal detection and management system.
- After communication, the concluded information is published in publicly available WHO-newsletters or scientific journals to prevent any further damage to the patients.
References:
Drug Regulatory Authority of Pakistan. (2025). Drug Regulatory Authority of Pakistan. Retrieved from https://www.dra.gov.pk/
Uppsala Monitoring Centre. (2025). uppsala monitoring cnetre. Retrieved from https://who-umc.org/