Migraine Attack Case Study: Micronutrient-Related Factors in Episodic to High-Frequency Migraine
 Patient Profile
Patient Profile
- Name: Mr. R
- Age: 41 years
- Sex: Male
- Occupation: Operations manager (high cognitive load, irregular work hours)
- Lifestyle: Physically inactive, high caffeine intake
- Sleep pattern: Early waking on weekdays with frequent sleep debt
 Chief Complaint
Chief Complaint
Recurrent headache episodes over the past 7 years, with progression from episodic to high-frequency migraine during the last 10 months.
 History of Present Illness
History of Present Illness
- Headache frequency: 10–12 days/month
- Pain characteristics:
- Throbbing, bilateral frontal or occipital
- Moderate to severe intensity
- Duration: 12–72 hours
- Associated features:
- Photophobia
- Phonophobia
- Reduced concentration
- Neck and jaw muscle tightness
- Post-headache lethargy
 Reported Triggers
Reported Triggers
- Sleep deprivation
- Prolonged fasting
- Excess caffeine followed by withdrawal
- Emotional stress
- Weather changes
 Past Medical History
Past Medical History
- Gastroesophageal reflux disease (GERD)
- Mild hypertension (diet controlled)
- No history of seizures, diabetes, or renal disease
 Medication History
Medication History
- Frequent use of:
- NSAIDs
- Caffeine-containing analgesics
- Occasional proton pump inhibitor use
- No regular migraine prophylaxis
 Dietary and Lifestyle History
Dietary and Lifestyle History
- Skips breakfast frequently
- Low intake of:
- Whole grains
- Legumes
- Nuts and seeds
- Rare fish consumption
- Minimal dairy intake
- Limited outdoor exposure
 Family History
Family History
- Father with history of chronic headache
- No family history of stroke or epilepsy
 Physical Examination
Physical Examination
- Blood pressure: 128/82 mmHg
- BMI: 26.1 kg/m²
- General examination: Mild cervical muscle tenderness
- Neurological examination: Normal
 Laboratory Investigations
Laboratory Investigations
Test | Patient Value | Reference Range |
Hemoglobin (Hb) | 13.0 g/dL | 13.5–17.5 g/dL |
Mean Corpuscular Volume (MCV) | 86 fL | 80–96 fL |
Serum Ferritin | 42 ng/mL | 30–400 ng/mL |
Vitamin B12 | 280 pg/mL | 200–900 pg/mL |
Serum Folate | 6.1 ng/mL | 4.0–20.0 ng/mL |
Plasma Homocysteine | 17.5 µmol/L | 5–15 µmol/L |
Vitamin B6 (PLP) | 16 nmol/L | 20–125 nmol/L |
Vitamin B2 (Riboflavin) | Below optimal | Adequate |
25-Hydroxy Vitamin D | 21 ng/mL | 30–100 ng/mL |
Serum Magnesium | 1.6 mg/dL | 1.7–2.4 mg/dL |
RBC Magnesium | Low | Normal |
Zinc | 58 µg/dL | 70–120 µg/dL |
Copper | 142 µg/dL | 70–140 µg/dL |
Omega-3 Index | 3.2% | ≥8% |
Fasting Glucose | 96 mg/dL | 70–99 mg/dL |
ALT (SGPT) | 24 U/L | 7–35 U/L |
AST (SGOT) | 22 U/L | 8–33 U/L |
Case-Based Questions
- Which micronutrient abnormalities in this patient are most strongly associated with progression toward high-frequency migraine?
- How might disturbances in one-carbon metabolism explain the elevated homocysteine despite apparently adequate folate levels?
- How could zinc deficiency and a high copper-to-zinc ratio influence neuroinflammation and headache susceptibility?
- What role might a low omega-3 index play in central sensitization and trigeminovascular activation?
- How can frequent NSAID and caffeine use interact with micronutrient depletion and migraine chronification?
- Which lifestyle and dietary factors in this case are likely contributing to impaired micronutrient absorption or increased requirements?
- What safety considerations should guide long-term nutritional interventions in a middle-aged male with GERD and intermittent PPI use?
- Based on this case, how would you differentiate a nutritional-metabolic migraine phenotype from medication-overuse headache?
 Pharmacist’s Workup:
Pharmacist’s Workup:
- Which micronutrient abnormalities in this patient are most strongly associated with progression toward high-frequency migraine?
In order to find out the micronutrient abnormalities, we first need to look at the lab report of the patient.
Test | Patient Value | Reference Range |
Vitamin B6 (PLP) | 16 nmol/L | 20–125 nmol/L |
Vitamin B2 (Riboflavin) | Below optimal | Adequate |
25-Hydroxy Vitamin D | 21 ng/mL | 30–100 ng/mL |
Serum Magnesium | 1.6 mg/dL | 1.7–2.4 mg/dL |
RBC Magnesium | Low | Normal |
Zinc | 58 µg/dL | 70–120 µg/dL |
Copper | 142 µg/dL | 70–140 µg/dL |
Omega-3 Index | 3.2% | ≥8% |
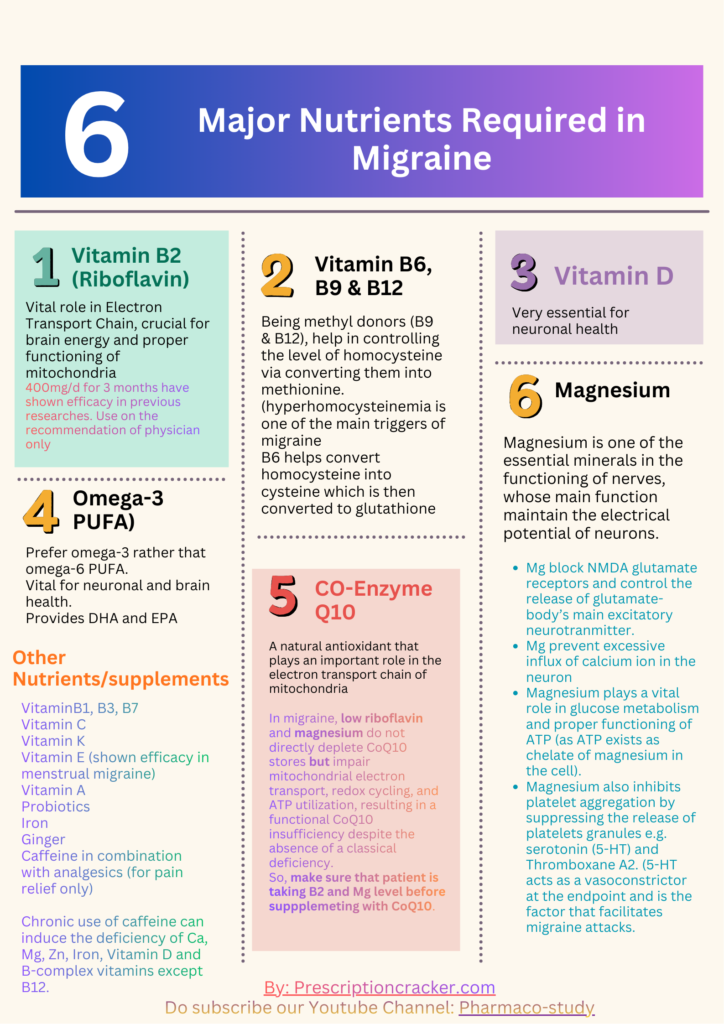
By looking at the lab report we come to known that the patient is deficient in Vitamin B6, B2, Serum magnesium, RBC magnesium, Omega-3, Zn, and Vitamin D, while there is excess of copper present in patient’s body.
Almost all of them have significant role in maintaining the brain health and preventing the attack of migraine however omega-3, Vitamin B2 and Mg are among the substantial of all, whose abnormalities can lead towards the progression of high-frequency migraine.
Before moving forward, let’s look at the role of Vitamin B2 and Mg in maintaining brain health (The role and importance of omega-3 is discussed in question no. 4):
Vitamin B2:
Riboflavin is one of the most crucial vitamin due to its anti-inflammatory, antioxidant, anti-nociceptive, & anti-aging properties. Its deficiency is often linked with neuroinflammation and disruption in brain energy due to mitochondrial dysfunction – the 2 main trigger causes of migraine attack.
Riboflavin is one of the important components of oxidative metabolism due to its role as the precursor to coenzymes in mitochondria:
- flavin mononucleotide (FMN) – a key component of complex-I, NADH dehydrogenase in ETC).
- flavin– adenosine–dinucleotide (FAD) – Component of succinate dehydrogenase in Krebs cycle.
Magnesium:
Magnesium is one of the most essential mineral required for the proper functioning of neurons, whose main role is to maintain the electrical potential of neurons in the following ways:
- Mg block NMDA glutamate receptors and control the release of glutamate-body’s main excitatory neurotranmitter.
- Mg prevent excessive influx of calcium ion in the neuron.
Magnesium plays a vital role in glucose metabolism and proper functioning of ATP (as ATP exists as chelate of magnesium in the cell).
Magnesium also inhibits platelet aggregation by suppressing the release of platelets granules e.g. serotonin (5-HT) and Thromboxane A2.
Magnesium deficiency in the brain leads to platelet aggregation and the release of glutamate, resulting in the formation of 5-hydroxytryptamine (5-HT). 5-HT acts as a vasoconstrictor at the endpoint and is the factor that facilitates migraine attacks.
Low level of magnesium causes the removal of physiological brake on NMDA receptors and result in excessive glutamate activity triggering cortical spreading depression (CSD) of migraine aura along.
Mg deficiency also leads to influx of calcium ion in the neurons triggering the release of stored neurotransmitters especially CRGP and substance P, contributing to migraine pain and other characteristic symptoms of migraine.
ATP/energy production also impairs due to the deficiency of magnesium, leaving the neuron energy deficient during the period of stress.
Magnesium is also involved in the synthesis of phospholipids and the embedment of proteins into the phospholipid membrane, and is therefore essential to membrane stabilization.
Thus, the patient under study needs Vitamin B2, and Mg on immediate basis along with other deficient nutrients.
FAQ: Why Serum Magnesium Can Be “Normal” in Migraine
- <1% of total body magnesium is in serum
- Intracellular and neuronal Mg levels may be low despite normal labs
- Migraine is associated with:
- Reduced ionized Mg²⁺
- Impaired Mg transport into cells
➡️ This explains why migraine is linked to functional magnesium deficiency, not always lab-detectable deficiency.
- How might disturbances in one-carbon metabolism explain the elevated homocysteine despite apparently adequate folate levels?
Homocysteine levels are regulated through one-carbon metabolism, a group of interconnected pathways that transfer single-carbon units needed for normal cellular function. In this system, homocysteine is remethylated to methionine using folate-derived methyl groups with vitamin B12 as a cofactor, leading to the formation of S-adenosylmethionine (SAM), the body’s main molecule that donates methyl groups for DNA, histone, and neurotransmitter methylation. Vitamin B6, while not a direct methyl donor, supports homocysteine clearance through the transsulfuration pathway by converting it to cysteine, thereby preventing homocysteine accumulation and its neurotoxic effects.
Now if we look at patient’s lab report, we will find out that the patient has sufficient folate level but is deficient in vitamin B6. Its also noteworthy that vitamin b12 level is almost marginal in this patient. Vitamin B6 plays a vital role in the clearance of homocysteine through the process of transsulfuration now as the vitamin B6 is less than the required amount, it can be an eminent reason of high homocysteine level. Secondly, borderline vitamin B12 may limit efficient remethylation to methionine. Riboflavin deficiency further compromises MTHFR enzyme efficiency, reducing effective folate utilization at the cellular level. This is the reason that the patient has high level of homocysteine despite apparently adequate folate levels.
So, this patient needs vitamin B6 and B12 supplements along with improvement/incorporation of dietary sources of these vitamins, in order to maintain normal homocysteine level.
- How could zinc deficiency and a high copper-to-zinc ratio influence neuroinflammation and headache susceptibility?
Copper is an acute-phase reactant during inflammation. Throughout the systemic inflammation, levels of ceruloplasmin (a major copper-binding protein) rise due to excessive synthesis by liver which results in increasing serum copper concentration. While the Zinc levels tend to decrease during inflammation as zinc is redistributed into the liver and immune tissues in response to inflammatory cytokines like IL-6, lowering circulating Zn.
Consequences of elevated Cu/Zn ratio:
- Copper and zinc are cofactors for Cu-Zn superoxide dismutase (SOD), a major antioxidant enzyme.
- An imbalance in Cu and Zn disrupts SOD function, leading to oxidative stress, a key driver of inflammation.
Higher Cu/Zn ratio correlates with elevated CRP- an important marker of inflammation. So, High Cu/Zn ratio is suggestive of active inflammation, oxidative stress, or stress-related micronutrient redistribution.
Now let’s look at the whole mechanism of relationship between elevation of Cu/Zn ratio and neuroinflammation:
In migraine, the relationship between inflammation, neuroinflammation, and changes in the copper–zinc (Cu/Zn) ratio is bidirectional rather than linear, and this is crucial for correct interpretation. In some individuals, inflammatory processes begin outside the brain due to metabolic stress, nutritional deficiencies, hormonal fluctuations, or systemic immune activation. This peripheral inflammation leads to increased production of pro-inflammatory cytokines such as interleukin-6, which stimulates hepatic synthesis of ceruloplasmin and consequently raises circulating copper levels, while zinc is redistributed away from plasma and neural tissues. These inflammatory signals can influence the brain through cytokine penetration at vulnerable regions of the blood–brain barrier, endothelial activation, and neural immune signalling pathways. As a result, microglia (brain’s immune cells) become informed, trigeminovascular pathways are sensitized, and a migraine attack is triggered. In this scenario, an elevated Cu/Zn ratio reflects a systemic inflammatory state that contributes to neuroinflammation rather than merely responding to it.
In other migraineurs, particularly those with strong genetic predisposition, early onset, or aura, the process may originate within the brain itself. Intrinsic neuronal vulnerabilities such as mitochondrial inefficiency, impaired magnesium-dependent inhibition of NMDA receptors, or cortical hyperexcitability can initiate neuroinflammation directly. Microglial activation, cortical spreading depression, and CGRP release then act as central inflammatory events. These brain-derived inflammatory signals are communicated to the periphery through neuroendocrine and immune pathways, leading to a secondary acute-phase response in the liver. This response increases serum copper and lowers circulating zinc, resulting in an elevated Cu/Zn ratio. In this case, the altered Cu/Zn ratio is a downstream systemic marker of primary neuroinflammation, not its cause.
Importantly, once migraine becomes recurrent or chronic, these two pathways no longer remain distinct. Peripheral inflammation and central neuroinflammation form a self-reinforcing loop, where systemic inflammatory changes worsen neuronal excitability and brain inflammation further amplifies peripheral immune responses. The Cu/Zn ratio, therefore, should be understood not as evidence of where inflammation started, but as a biochemical signature of an ongoing neuro-immune interaction. In migraine, it reflects inflammatory tone, oxidative stress, and impaired immune regulation that can both drive and result from neuroinflammation, depending on the clinical context.
It has been shown that zinc supplementation can decrease the Cu/Zn ratio and CRP level. So, Zn supplementation could be considered in this patient along with careful monitoring of the Cu/Zn level.
- What role might a low omega-3 index play in central sensitization and trigeminovascular activation?
Firstly, we need to clear the positions of omega-3 and omega-6 polyunsaturated fatty acids (PUFA).
There are two vital types of polyunsaturated fatty acids (PUFAs) :
- Omega-3
- Omega-6 PUFAs
Both omega-3 and omega-6 PUFAs play substantial roles in cardiovascular, anti-inflammatory, metabolic, neurological, and psychiatric health.
Omega-3 PUFAs originate from alpha-linolenic acid (ALA), while omega-6 PUFAs come from linoleic acid (LA).
These are considered to be essential fatty acids because they must be obtained from the diet, as the human body cannot synthesize them independently. Alpha-linolenic acid (ALA) and linoleic acid (LA) undergo competitive metabolism through two distinct enzymatic pathways. In the liver, the process of desaturation, elongation, and β-oxidation reactions converts:
- ALA into EPA and docosahexaenoic acid (DHA),
- LA is transformed into Arachidonic acid (AA)
Omega-3 PUFAs offer cardiovascular, neurological, and psychiatric benefits due to their potent anti-inflammatory, anti-nociceptive, antioxidant, and neuromodulatory properties, which modulate neuroinflammation, pain transmission, enhance mitochondrial stability, and mood regulation.
While omega-6 PUFAs possess pro-inflammatory actions, through competitive metabolic processes. They act as pioneers for various bioactive lipid mediators, known collectively as oxylipins.
Despite the liver and brain’s capacity to convert ALA into DHA, the endogenous production of EPA and DHA in the brain is comparatively low, requiring their active uptake from the bloodstream. The modern diet, which typically contains up to 15 times more omega-6 PUFAs than omega-3 PUFAs, along with a common deficiency of DHA in the brain, results in the production of large amounts of lipid mediators derived from LA and AA, contributing to chronic inflammation and oxidative stress implicated in cardiovascular, metabolic, inflammatory, and neuropsychiatric disorders.

Specialized pro-resolving mediators (SPMs) are a class of PUFA derived lipid mediators. Different SPMs are derivatives from differing PUFA substrates:
- E-series resolvins (RvE1, RvE2, and RvE3) are derived from EPA
- D-series resolvins (RvD1, RvD2, RvD3, RvD4)
- Protectins (protectin D1, a.k.a. neuroprotectin D1 when formed in the nervous system)
- Maresins are derived from DHA
- Lipoxins are derived from AA.
SPMs have significant anti-inflammatory, analgesic, tissue regeneration and pro-resolving properties.
To conclude, omega-3 PUFAs decreases critical inflammatory mediators like TNF-α, cyclooxygenase-2/NO synthase, and IL-1β. These reductions are believed to alleviate neuroinflammation and neurogenic pain, crucial elements in migraine pathophysiology.
An omega-3 index of 3.2% indicates a marked deficiency in EPA and DHA, which are essential for resolving inflammation and stabilizing neuronal membranes. Low omega-3 status favors production of pro-inflammatory eicosanoids, increases CGRP signaling, and reduces endogenous pain-resolving mediators such as resolvins and protectins. This imbalance enhances trigeminovascular activation and central sensitization, contributing to prolonged and more frequent migraine attacks
- How can frequent NSAID and caffeine use interact with micronutrient depletion and migraine chronification?
Effect of NSAIDs on Micronutrient status:
Chronic use of NSAIDs can cause depletion of iron, Vitamin B9, Vitamin E, and Vitamin C, where iron deficiency being most often observed particularly due to NSAIDs associated GIT bleeding.
Caffeine:
Caffeine is a naturally occurring substance commonly found in the leaves, seeds and fruits of at least 63 plant species across the world. Caffeine belongs to the group of compounds known as methylxanthines.
The most commonly known sources of caffeine are coffee, cocoa beans, kola nuts and tea leaves. Caffeine is absorbed and passes quickly into the brain. Caffeine stimulates, or excites, the brain and nervous system.
Effect of Caffeine on Micronutrient status:
- For every 150 mg of caffeine ingested, about the amount in one cup of coffee, 5 mg of calcium is lost.
- Caffeine inhibits vitamin D receptors, which reduces the activity of vitamin D. Because vitamin D is crucial in the absorption and use of calcium in building bones, this could also decrease bone mineral density, consequently increasing the risk for osteoporosis
- Caffeine prevents the absorption of iron, which is vital for RBCs production. Coadministration of caffeine and iron containing food/drink can decrease the absorption of the iron by 80% as per Nutrition Desk Reference. So, any beverage containing caffeine should be separated from iron-containing foods or supplements by at least one hour
- Caffeine possesses mild diuretic effect, which can increase the urination. Water soluble vitamins, such as the B-vitamins, can be depleted as a result of the fluid loss. In addition, caffeine restricts the metabolism of thiamine (vitamin B1). The only exception to this rule appears to be vitamin B12. It’s because, caffeine induces the production of stomach acid, which actually helps the body absorb B12.
- Caffeine may reduce the absorption of manganese, zinc and copper. It also increases the excretion of the minerals magnesium, potassium, sodium and phosphate. It is also indicated that caffeine interferes with the action of vitamin A.
To conclude, the imbalance of micronutrient status by NSAIDS and caffeine can contribute to the initiation of migraine headaches and its chronification.
Now, if we look at the consumption pattern of caffeine and NSAIDs in the patient under study, we will come to know that caffeine intake in this patient is extremely high which obviously can contribute to the initiation and chronification of the migraine, as a result of underlying micronutrient deficiencies.
The major intervention in this case should be the caffeine withdrawal along with shifting the patient from caffeine base analgesic to the single analgesics.
- Which lifestyle and dietary factors in this case are likely contributing to impaired micronutrient absorption or increased requirements?
- Physically inactive, high caffeine intake
- Prolonged fasting
- Excess caffeine followed by withdrawal
- Frequent use of:
- NSAIDs
- Caffeine-containing analgesics
- Skips breakfast frequently
- Low intake of:
- Whole grains (rich in B-complex vitamins)
- Legumes (rich in protein and B-complex vitamins)
- Nuts and seeds (rich in minerals like calcium, magnesium and other minerals along with protein and omega-3 PUFA)
- Rare fish consumption (good source of omega-3 PUFA)
- Minimal dairy intake (rich in calcium, Mg and vitamin D)
- Limited outdoor exposure (Create the vulnerability of Vitamin D deficiency)
Physical inactivity worsens insulin sensitivity and mitochondrial biogenesis. High cognitive load and sleep debt further raise micronutrient requirements and stress neuro-energetic systems. These dietary and lifestyle factors are the most likely contributing to impaired micronutrient absorption or increased requirements
- What safety considerations should guide long-term nutritional interventions in a middle-aged male with GERD and intermittent PPI use?
A middle-aged person who is experiencing the symptoms of GERD and using PPIs intermittently should adopt following measures:
- Avoid caffeine-based food and beverages
- Add dairy products like milk and yogurt in moderate amount (avoid excessive usage)
- Take NSAIDs only when needed
- Daily 30minutes of exercise
- Add whole grains, nuts, and legumes etc but no to add excessive spices in them
- Drink appropriate amount of water to prevent dehydration.
- Avoid smoking
- Supplementation should prioritize bioavailable forms (e.g., magnesium glycinate or threonate, methylated B vitamins if needed).
- Vitamin D should be corrected gradually with monitoring of calcium levels.
- Based on this case, how would you differentiate a nutritional-metabolic migraine phenotype from medication-overuse headache?
The case under study seems nutritional-metabolic migraine rather than medication overuse headache because the patient has much nutrition base discrepancies (low magnesium, B6, B2, zinc, omega-3, elevated homocysteine, high Cu/Zn ratio) that independently explain neuronal hyperexcitability and migraine progression. while Medication-overuse headache is primarily defined by headache worsening due to analgesic dependence and typically improves rapidly after withdrawal, whereas this patient’s profile suggests an underlying metabolic vulnerability that requires correction of micronutrient status alongside rational medication use.
References:
Mayans, L., & Walling, A. (2018). Acute migraine headache: Treatment strategies. American Family Physician, 97(4), 243–251. https://www.aafp.org/pubs/afp/issues/2018/0215/p243.html
Hajhashemy, Z., Golpour-Hamedani, S., Eshaghian, N., Sadeghi, O., Khorvash, F., & Askari, G. (2024). Practical supplements for prevention and management of migraine attacks: A narrative review. Frontiers in Nutrition, 11, Article 1433390. https://doi.org/10.3389/fnut.2024.1433390
Schneider, T., Caviezel, D., Ayata, C. K., Kiss, C., Niess, J. H., & Hruz, P. (2020). The copper/zinc ratio correlates with markers of disease activity in patients with inflammatory bowel disease. Crohn’s & Colitis 360, 2(1), otaa001. https://doi.org/10.1093/crocol/otaa001
Chen, T.-B., Yang, C.-C., Tsai, I.-J., Yang, H.-W., Hsu, Y.-C., Chang, C.-M., & Yang, C.-P. (2024). Neuroimmunological effects of omega-3 fatty acids on migraine: A review. Frontiers in Neurology, 15, Article 1366372. https://doi.org/10.3389/fneur.2024.1366372
National Academies of Sciences, Engineering, and Medicine. (2019). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK507271/
Turnlund, J. R., & Keen, C. L. (2018). Micronutrient status and metabolic effects in chronic diseases. Nutrition in Clinical Practice, 33(5), 673–687. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6109862/
Johnson, R. J., Sánchez Lozada, L. G., Andrews, P. F., & Rodriguez-Iturbe, B. (2022). Dietary factors and kidney health: A narrative review. Nature Reviews Nephrology, 18(3), 123–139. https://doi.org/10.1038/ncpneph0522
Lumen Learning. (n.d.). Electron transport chain. Retrieved January 6, 2026, from https://courses.lumenlearning.com/wm-biology1/chapter/reading-electron-transport-chain/
Temesgen, W., & Gebremariam, S. (2015). Effects of caffeine on health and nutrition: A review. https://www.researchgate.net/publication/279923885_Effects_of_caffeine_on_health_and_nutrition-A-Review
Copper/Zinc ratio correlates with markers of disease activity in patients with inflammatory bowel disease. Crohn’s & Colitis 360. https://academic.oup.com/crohnscolitis360/article-abstract/2/1/otaa001/5714917