On December 18, 2025 – DRAP Recall Falsified/Spurious/ unregistered Tramadol tablets & Substandard betahist 16mg & Glitric 2.6mg Tablets
DRAP Recall Alert No: I/S/12-25-122
Action Date: 18 December 2025
Key Information
The Drug Regulatory Authority of Pakistan (DRAP) has issued a rapid alert based on findings reported by the Central Drugs Laboratory (CDL), Karachi.
Drug samples were submitted for testing by the National Task Force (NTF).
The tested products were declared unregistered, spurious, and/or falsified.
Identified Products
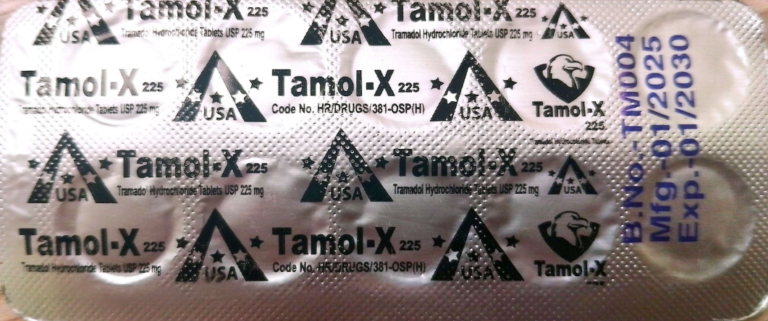
Tablet TAMOL-X 225 mg
Declared as unregistered and falsified
- Batch no: TM004
Not registered with DRAP
Tramaking 250 mg Tablets
Declared as unregistered and falsified
- Batch no: TRD379
Not registered with DRAP
Namadol 225 mg Tablets
Declared as spurious, falsified, and unregistered
- Batch no: TRD2025
Laboratory analysis also confirmed absence of the claimed Active Pharmaceutical Ingredient (Tramadol HCl)
Manufacturer Details (as per labeling)
M/s Ciba Pharmaceuticals (Pvt.) Ltd.
A-371, Nooriabad, Main Super Highway, Karachi
Drug Manufacturing Licence (DML): 000825
Regulatory Status
None of the above products are registered with DRAP.
These products have not been evaluated or approved for:
Safety
Quality
Efficacy
Risk Statement
Use of these products poses a serious public-health risk due to:
Unknown or inconsistent composition
Uncertain strength and quality
Illegal manufacturing and marketing
Potential health consequences include:
Therapeutic failure
Poor or uncontrolled pain management
Opioid withdrawal symptoms
Unexpected toxicity or overdose
Respiratory depression
Serious adverse drug reactions
Populations at Higher Risk
Patients with moderate to severe pain
Individuals with chronic pain conditions
Post-operative patients
Persons misusing or dependent on opioid analgesics
Elderly patients
Patients with hepatic, renal, or respiratory disorders



DRAP Recall Alert No: II/S/12-25-123
Action Date: 18 December 2025
Target Audience
National Regulatory Field Force
Healthcare professionals:
Physicians
Pharmacists
Nurses working in hospitals and clinics
General public
Problem / Issue
Drugs, Water & Food Testing Laboratory, Government of Gilgit-Baltistan, reported quality testing results.
Samples of certain drug products were analyzed and declared substandard.
Therapeutic Goods Affected
Glitric Tablets 2.6mg (Prolonged Release)
Each tablet contains: Glyceryl Trinitrate 2.6 mg
Registration No.: 119607
Batch Nos.: 25H261 and 25H262
Quality Findings:
Batch 25H261 declared substandard based on assay and dissolution test failure
Batch 25H262 declared substandard based on assay test failure
Manufacturer:
M/s Linta Pharmaceuticals
Plot #03, Street S-5, National Industrial Zone, Rawat, Islamabad
Drug Manufacturing Licence (DML): 000810
Betahist 16mg Tablets
Each tablet contains: Betahistine Dihydrochloride 16 mg
Registration No.: 091595
Batch No.: 25H249
Quality Findings:
Declared substandard due to physical defects, including:
Softening of tablets
Cracking
Rough, orange-peel–like surface texture
Tablets easily breaking
Tablets turning into powder upon finger pressure
Risk Statement:
Identified quality defects in the affected products may lead to therapeutic failure and unpredictable dosing.
Glitric 2.6 mg (Glyceryl Trinitrate prolonged-release tablets):
Assay and dissolution failures can result in inadequate drug release.
Glyceryl Trinitrate has a narrow and sensitive therapeutic window.
Subtherapeutic dosing may precipitate:
Angina attacks
Serious ischemic cardiovascular events
Elderly patients and those with ischemic heart disease are at higher risk.
Betahist 16 mg (Betahistine tablets):
Severe physical instability may compromise dose accuracy and efficacy.
Loss of therapeutic effect may occur in patients suffering from:
Vertigo
Balance and vestibular disorders
Population Most Likely to be Affected
Patients with chronic cardiovascular diseases
Patients with vestibular and balance disorders
Elderly patients
Patients with comorbid conditions
Actions Taken
The Regulatory Field Force has been instructed to conduct surveillance and seize the above-mentioned unregistered/falsified products.
Pharmacists, chemists, distributors, and healthcare professionals must urgently inspect their stocks. Details of suppliers of such products should be reported to the Regulatory Field Force (DRAP and Provincial Drug Control Departments) to ensure removal from circulation.
Distributors and pharmacies are advised to be vigilant and report any suspected batch of the product(s) in the supply chain to the DRAP using the online form, or by Email at gsms@dra.gov.pk.
Guidance for Healthcare Professionals
Any adverse events or quality concerns linked to these medicines should be reported to the National or Provincial Pharmacovigilance Centres via the Adverse Event Reporting Form or through the official online portal.


Guidance for Consumers
Consumers must avoid these products. If anyone experiences health problems after using them, they should immediately consult a healthcare provider and report the incident to DRAP / the National Pharmacovigilance Centre.
Always purchase therapeutic goods only from licensed pharmacies or authorized outlets. Carefully check the authenticity and condition of products, and consult a pharmacist or healthcare professional in case of doubt.
References :
Drug Regulatory Authority of Pakistan. (2025, December 18). 122. Rapid alert – Falsified / spurious / unregistered Tramadol tablets. https://www.dra.gov.pk/safety_info/product_recalls/recall_alerts/122-rapid-alert-falsified-spurious-unregistered-tramadol-tablets/ Defense Housing Authority
Drug Regulatory Authority of Pakistan. (2025, December 18). 123. Recall alert (Class-I) – Drug products (human) substandard – Gilgit Baltistan. https://www.dra.gov.pk/safety_info/product_recalls/recall_alerts/123-recall-alert-class-i-drug-products-human-substandard-gligit-baltistan/


