Table of Content
- Topical Steroids
- Topical steroids available potencies and formulations
- Conditions responsive to topical steroids
- Maximum duration for the treatment of Topical steroids in different conditions
- Hypertrichosis
- Difference between hypertrichosis and hirsutism
- Types of hypertrichosis
- Medication that causes hypertrichosis
- Association between topical steroids and hypertrichosis
- Management of hypertrichosis associated with topical steroids
- Abuse of topical steroids as a fairness creams
- Abuse of of topical steroids as therapeutic agent and Nappy rash cream
- Key Takeaways
- References
Steroids whether oral or topical have been marketed and used by a large population for various purposes for several decades. Though the use of oral steroids have been largely controlled by the regulatory authorities and is strictly available on the prescription, the topical steroids are easily available over-the-counter especially in the developing countries which has resulted in its extreme abuse for the self-medication and beautification purposes.
But one of its major drawback, that is associated with the long term use is unwanted growth of hair on the area of application (Hypertrichosis), which is often ignored due to the lack of awareness. This side effect can extensively affect the appearance of a person.
This side effect is uncommon in the patients who uses topical steroids as per prescribed duration and do not exceed the prescribed limit. However those who uses these topical steroids as a part of self-medication for dermatological condition often disregard the duration limit and thus suffers from this side effect.
Likewise the majority of the lay people uses these topical steroids for beautification at home and at salons. Similarly they also disregarded the duration limit and thus end up having opposite of what they wanted in the form of unwanted hair on their skin.

Topical steroids
Topical steroids are the topically applied pharmaceutical preparation of steroids used for the treatment of eczema, dermatitis, psoriasis, vitiligo and other inflammatory disorders of skin. Topical steroids are available in the different formulation like ointments, creams, solutions and lotions etc. Most commonly available topical steroids and marketed formulations include:
Potency Class of steroids | Agent (Concentration) | Formulations |
I (Super–high)
| Betamethasone dipropionate 0.05% (augmented) | Ointment, lotion, gel |
| Clobetasol propionate 0.05% | Ointment, cream, lotion, gel, foam, solution, shampoo, spray |
| Fluocinonide 0.1% | Cream |
| Flurandrenolide 4 µg/cm² (tape) | Tape |
| Halobetasol propionate 0.05% | Ointment, cream |
II (High) | Amcinonide 0.1% | Ointment, cream |
| Betamethasone dipropionate 0.05% | Ointment |
| Betamethasone dipropionate 0.05% (augmented) | Cream |
| Desoximetasone 0.25% | Ointment, cream |
| Desoximetasone 0.05% | Gel |
| Diflorasone diacetate 0.05% | Ointment |
| Fluocinonide 0.05% | Ointment, cream, gel, solution |
| Halcinonide 0.1% | Ointment, cream, solution |
III–V (Medium) | Amcinonide 0.1% | Lotion |
| Betamethasone dipropionate 0.05% | Cream, lotion |
| Betamethasone valerate 0.1%–0.12% | Ointment, cream, lotion, foam |
| Diflorasone diacetate 0.05% | Cream |
| Fluocinolone acetonide 0.025% | Ointment, cream |
| Flurandrenolide 0.05% | Ointment, cream, lotion |
| Fluticasone propionate 0.05–0.005% | Cream, lotion, ointment |
| Hydrocortisone butyrate 0.1% | Ointment, cream, lotion, solution |
| Hydrocortisone valerate 0.2% | Ointment, cream |
| Mometasone furoate 0.1% | Ointment, cream, lotion, solution |
| Triamcinolone acetonide 0.1–0.5% | Ointment, cream, lotion, spray |
VI–VII (Low) | Alclometasone dipropionate 0.05% | Ointment, cream |
| Desonide 0.05% | Ointment, cream, lotion, gel, foam |
| Fluocinolone acetonide 0.01% | Cream, solution, oil |
| Hydrocortisone 1–2.5% | Ointment, cream, lotion, spray |
| Triamcinolone acetonide 0.025% | Ointment, cream, lotion |
Topical corticosteroids are also marketed in combination with antibacterial (Fucidin-h®, Betnovate-N® etc.) and antifungal creams (Hydrozole®).
The effect of the topical steroids depends upon the potency of the steroid in the formulation used. Here is the table defining the effect of potency on the related disease treated.
Steroids’s potency | Conditions Responsive to Therapy |
High-potency (Classes I & II) | – Alopecia areata – Atopic dermatitis (resistant) – Bullous pemphigoid – Discoid lupus – Dyshidrotic eczema – Hyperkeratotic eczema – Labial adhesion – Lichen planus – Lichen sclerosus (skin) – Lichen simplex chronicus – Melasma – Nummular eczema – Poison ivy (severe) – Psoriasis – Vitiligo |
Medium-potency (Classes III–V) | – Anal inflammation (severe) – Asteatotic eczema – Atopic dermatitis – Dermatitis (severe) – Infantile acropustulosis – Intertrigo (severe, short term) – Lichen sclerosus (vulva) – Nummular eczema – Scabies (after scabicide) – Seborrheic dermatitis – Stasis dermatitis |
Low-potency (Classes VI–VII) | – Diaper dermatitis – Dermatitis (eyelids) – Dermatitis (face) – Intertrigo – Perianal inflammation – Phimosis |
Maximum duration of the treatment:
Maximum duration of the treatment depends upon the potency of the topical steroid used. Such as:
- Maximum duration of treatment for using super high potency corticosteroids is 3 weeks.
- Maximum duration of treatment for using high potency corticosteroids is 12 weeks.
- Maximum duration of treatment for using medium potency corticosteroids is 12 weeks.
- Maximum duration of treatment for using low potency corticosteroids is not specified.
Hypertrichosis:
Hypertrichosis is defined as the excessive growth of hair in the areas of the body where the growth of hair is abnormal or previously not much dense in nature.
Hypertrichosis occurs as a result of local follicle stimulation. The grown hair looks more like that of vellus hair (Soft and fine) in the area with no previous hair growth and if that area already consist of vellus hair then upon the occurrence of hypertrichosis the vellus hair converted to the terminal hair.
The pathophysiological feature behind the hypertrichosis is the prolongation of the anagen phase (growth phase) and shortening of telogen and exogen phase causing faster hair regeneration.
Its risk of occurrence is almost same in both male and females and can occur at any age.
It is different from the hirsutism which is highly regulated by the disturbance of the hormone especially due to excess of androgen hormone. And the grown hair are usually terminal hair (Thick and coarse). It also should be noted that unlike hypertrichosis, hirsutism is more common in females and occurs more commonly occurs during the period of hormonal changes like puberty, pregnancy, or menopause.
Medication that causes hypertrichosis
Hypertrichosis can occur in the individuals who are taking the following medications, the development of hypertrichosis depends upon the duration of the treatment and often stops upon the discontinuation of that medication.
Medication | Onset | Localization |
Fenoterol | Within 1 month | Face and trunk |
Streptomycin | Within 1 month | Face, trunk, and extremities |
Benoxaprofen | Within 2 months | Face, back |
Corticosteroids | Within 10 months | Face and extremities |
Phenytoin | Within 3 months | Trunk, face, and extensor surfaces |
Cetuximab | Within 3 months | Scalp, eyelashes, and face |
Panitumumab | Within 3 months | Cheeks, upper lip, and chin |
Erlotinib | Within 5 months | Face and eyelashes |
Gefitinib | Within 7 months | Eyelashes |
Tacrolimus | Within 2 months | Face and eyelashes |
Cyclosporine | Within 6 months | Face, eyelashes, back |
Penicillamine | Within 2 months | Trunk and limbs |
Crisaborole | Within 2 months | Extremities |
Oral minoxidil | Within 2 months | Face and extremities |
Topical minoxidil | Within 3 months | Face and extremities |
Diazoxide | Within 1 month | Scalp and face |
Prostaglandin E1 | Within 3 months | Face and eyelashes |
Medication | Onset | Localization |
Fenoterol | Within 1 month | Face and trunk |
Association between Topical steroids and Hypertrichosis:
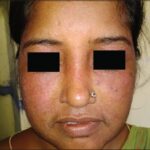
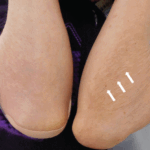
Among the other side effects of topical steroids like atrophy, acneiform eruption, hypopigmentation, delayed wound healing and rosacea, hypertrichosis is one of the most significant effect that the topical steroids produce that not only affects the physical appearance of the patient but also depress a person psychologically.
A number of the patients have reported the effect of hypertrichosis on the area of the application of corticosteroid cream.
The mechanism behind the growth of hair associated with the use of topical steroids is the prolongation of the anagen phase of the hair which is the main growth phase and shortening of the telogen and exogen phase.
This side effect of topical steroids is more commonly observed in the following conditions:
- Potent or very potent (Class I–II) corticosteroids
- Prolonged use, especially under occlusion or on thin skin (e.g., face, genital area)
- Children, due to increased percutaneous absorption.
Some places, for example the face, the groin and under the arms or breasts are more prone to side effects than other.
Management
Management of hypertrichosis associated with the use of topical steroids involved the following steps:
- Discontinuation or reduction of corticosteroid use
- Switching to lower-potency steroids
- Cosmetic options: trimming, bleaching, laser hair removal (for persistent cases)
Abuse of Topical steroids- As fairness Creams:
The use of topical steroids for the purpose of achieving fairness and beautification is something that is increasing day by day. The topical steroids are not only being misused by the general public and at beauty salons but also some local manufacturers of beauty creams are using these topical steroids for enhancing the effects of their product without clear declaration of the presence of steroids upon the label of the products.
A number of side effects have been seen with the long term use of topical steroids as a fairness cream like:
- Pustules
- Acneform lesions
- Erythematous papules
- Dryness
- Photosensitivity
- Hypertrichosis
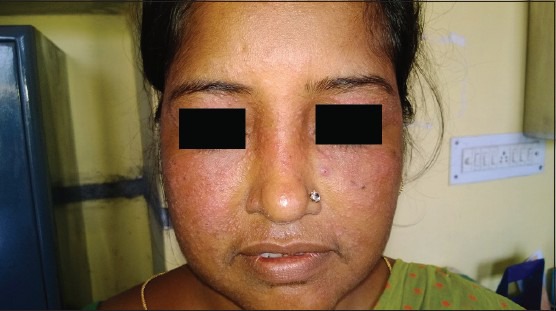
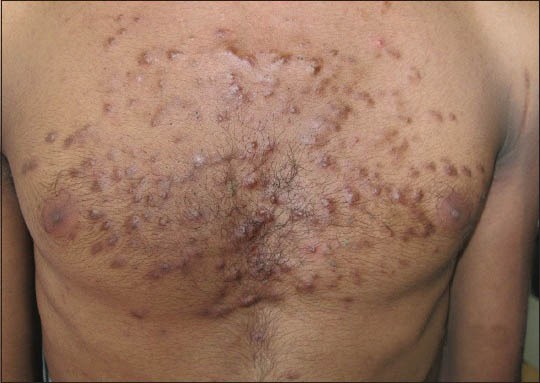
The abuse of topical steroids is not just limited to the fairness products but it also involves the unawareness regarding the duration limit of prescribed topical steroids such prescribing the steroids in combination with antifungal agents for tinea corporis without clearly informing the maximum duration for use and tapering off method to the patient can leads to excessive use of such creams for even years, ending up with having truncal acne or any other disorder.
Likewise, the use of the most famous combination of steroid i.e. hydrocortisone + clotrimazole combination mostly marketed by the name of Hydrozole® or Creamazole® as a general nappy rash cream, is increasing day by day. Though it is often recommended in case of inflammatory fungal infection in babies for a maximum duration of 1 week, it can’t be regarded as a day to day use nappy rash cream for babies as it possesses a significant risk for adrenal suppression in babies.
Key takeaways:
- The association between the use of Topical steroids and hypertrichosis is significant
- Hypertrichosis is a documented but reversible side effect of topical corticosteroids
- It is dose- and duration-dependent and often resolves upon stopping treatment
- Recognizing and educating patients about this side effect is important to improve compliance and avoid unnecessary concern
- Topical steroids should be use only on the prescription of a registered medical practitioner for only a specified time as directed.
Reference:
Ghosh, A., Sengupta, S., Coondoo, A., & Jana, A. K. J. I. j. o. d. (2014). Topical corticosteroid addiction and phobia. 59(5), 465-468.
Surber, C., & Höhne, C. (2025). Hair-follicle density reduction in corticosteroid use prolongs anagen-to-telogen transition, causing faster hair regeneration. Journal Title, Volume(Issue), pages. https://doi.org/10.1016/j.jid.2025.01.013
Smith, J. A., & Doe, R. L. (2002). Localized hypertrichosis following topical corticosteroid therapy: A case series. Journal of the American Academy of Dermatology, 47(5), 715–719. https://doi.org/10.1016/S0190-9622(02)61504-X
Coondoo, A., Phiske, M., Verma, S., & Lahiri, K. (2021). Safe use of topical corticosteroids: Clinical guidelines. American Family Physician, 103(5), 337–348. https://www.aafp.org/pubs/afp/issues/2021/0315/p337.html
DermNet NZ. (n.d.). Topical corticosteroids. DermNet NZ. https://dermnetnz.org/topics/topical-steroid
Gupta, A. K., & Chow, M. (2019, August 1). Comparative efficacy of topical treatments in dermatology. American Family Physician, 100(3), 168–176. https://www.aafp.org/pubs/afp/issues/2019/0801/p168.html
Minnesota Department of Health. (n.d.). Tested products summary. https://www.health.state.mn.us/communities/environment/skin/docs/testedprds.pdf
British Association of Dermatologists. (n.d.). Topical corticosteroids Patient Information Leaflet. https://www.bad.org.uk/pils/topical-corticosteroids/
Thomas, S., & Green, G. (2022). Evaluating topical steroid side effects: Clinical overview. Cleveland Clinic Journal of Medicine, 89(2), 71–78. https://www.ccjm.org/content/ccjom/89/2/71.full.pdf
Johnson, T. (2005). Hypertrichosis after topical corticosteroid use: Clinical observations. Journal of the American Academy of Dermatology, 53(3), 491–495. https://doi.org/10.1016/S0190-9622(05)00255-0
Patel, S., & Lee, A. (2013). Mechanisms of hair growth stimulation following steroid exposure. International Journal of Dermatology, 52(7), 887–895. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171913/
Miller, R. H. (2019). In: Topical steroids. In S. Skinner & B. K. White (Eds.), StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK531462/
Ando, Y., Ono, S., Ono, Y., & Miura, Y. (2022, February 1). Hypertrichosis and topical corticosteroid use. Cleveland Clinic Journal of Medicine, 89(2), 71–72. https://doi.org/10.3949/ccjm.89a.21048
Disclaimer:
This article is for informational and educational purposes only. It does not substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare provider before starting any medication.